R-SENS HANDHELD

- Portable system with Point and Shoot features
- Detection of suspicious content like narcotics or hazardous materials within 30 seconds for emergency response opportunity
- Detection of chemical substances by using unique fingerprints in the Raman spectra
- Ability to identify, control and verify the chemical composition of the sample
- Customer specific design for goal-directed marketing
What is Raman Spectroscopy ?
Raman spectroscopy is a form of vibration spectroscopy and provides information on molecular vibrations that can be used for sample identification and quantitative analysis. The method comprises that sending a monochromatic light source (i.e. laser) onto the sample surface and analyzing the scattered light.
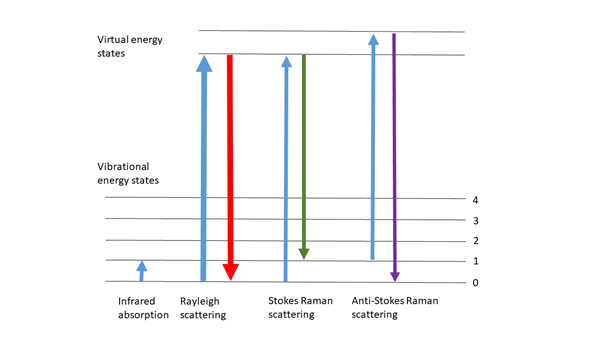
When light is scattered by matter, almost all of the scattering occurs elastically (Rayleigh scattering) and its energy does not change. However, a very small percentage of scattering occurs inelastically. In this case, the scattered light has different energy than the incoming light. This inelastic scattering of light was theoretically predicted by Adolf Smekal in 1923, and was first observed experimentally by Chandrasekhara Venkata Raman in 1928. Therefore, this inelastic scattering is called Raman scattering (Raman effect).
Raman scattering is a spectroscopic technique that complements infrared absorption spectroscopy. Raman offers several advantages over mid-IR and near IR spectroscopy:
- Sample preparation is minimal or not required at all.
- Water is a poor dispenser - no special accessories are required for measuring aqueous solutions.
- Water and CO2 vapor are very weak dispersants- No need to remove them from the sample.
- In most cases cheap glass sample containers are ideal.
- Fiber optic (up to 100 meters length) available for remote analysis.
- Raman bands can be easily associated with the chemical structure because of basic modes are measured.
- Raman spectra are "cleaner" than mid-IR spectra.
- Raman bands are narrower and overtone / combination bands are generally weak.
- Standard spectral range reaches below 400 cm-1 and this technique is ideal for both organic and inorganic species.
- Raman spectroscopy can be used for measure bands of symmetrical bonds that are weak in an infrared spectrum (i.e., -S-S-, -C = S-, -C = C-).
In Raman spectroscopy, samples may be in the following forms,
• Solid (particle, pellet, powder, film, fiber)
• Liquid (gel, paste)
• Gas


